A novel symmetrically multifunctionalized dodecamethoxy-cycloparaphenylene: synthesis, photophysical, and supramolecular properties†
Abstract
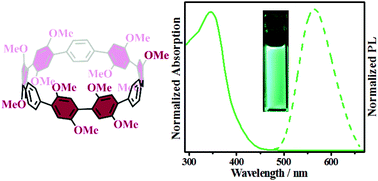
In the present study, we report the synthesis of a novel symmetrically multifunctionalized cycloparaphenylene (CPP), dodecamethoxy-[9]CPP (ring[9]arene), through nickel-mediated macrocyclization and subsequent reductive aromatization reactions. The electron-donating methoxy groups were introduced onto the curved precursor at an early stage to avoid the problem of non-selective functionalization of CPPs. Compared to [9]CPP, the present dodecamethoxy-[9]CPP shows a significant red shift (>73 nm) in the fluorescence spectrum. In addition, theoretical calculations revealed its increased torsion angles and ring strain (71.01 kcal mol−1) compared with its unsubstituted counterpart.


 Please wait while we load your content...
Please wait while we load your content...