5-Methylene N-acyl dihydropyridazinium ions as novel Mannich-type acceptors in 1,4 additions of nucleophiles†
Abstract
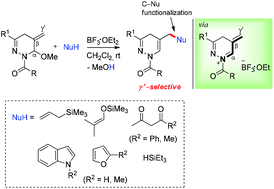
1,4,5,6-Tetrahydropyridazine featuring an iminium cation flanked by an exocyclic double bond was successfully employed as an N-acyliminium acceptor. Thus, in the presence of a Lewis acid (BF3·OEt2), generation and in situ interception of 5-methylene N-acyl dihydropyridazinium species with a range of various nucleophiles afforded diversified and functionalized 5-methylenyl-1,4-dihydropyridazine derivatives in good yields with excellent γ′-regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...