Asymmetric ring-opening of cyclopropyl ketones with β-naphthols catalyzed by a chiral N,N′-dioxide–scandium(iii) complex†
Abstract
A catalytic asymmetric ring-opening reaction of cyclopropyl ketones with β-naphthols has been accomplished. In the presence of a chiral N,N′-dioxide/scandium(III) complex, a series of aromatic or vinyl substituted cyclopropyl ketones reacted with 2-naphthols, providing efficient access to chiral β-naphthol derivatives in good yields (up to 99%) with good enantioselectivities (up to 97% ee). Notably, using the mixture of 2-naphthol substrates did not affect the efficiency of this catalytic system.
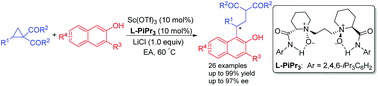


 Please wait while we load your content...
Please wait while we load your content...