Rh(iii)-Catalyzed directed C–H carbenoid coupling reveals aromatic bisphosphonates inhibiting metallo- and Serine-β-lactamases†
Abstract
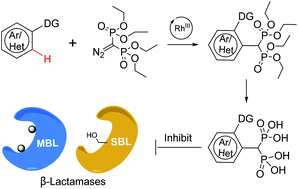
We report the highly efficient and scalable Rh(III)-catalyzed C–H coupling of arenes with diazo-methylene-diphosphonates to give structurally diverse aromatic bisphosphonates in good to excellent yields. These bisphosphonates are one of the very few classes of inhibitors acting against the mechanistically distinct metallo-β-lactamases and serine-β-lactamases and thus represent good starting points for the development of broad spectrum β-lactamase inhibitors.


 Please wait while we load your content...
Please wait while we load your content...