Iron/zinc-catalyzed benzannulation reactions of 2-(2-oxo-alkyl)benzketones leading to naphthalene and isoquinoline derivatives†
Abstract
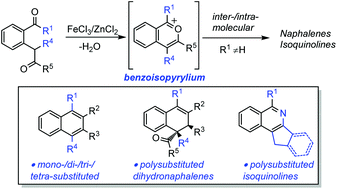
An efficient method for the synthesis of poly-substituted naphthalene derivatives via Fe(III)-catalyzed [4 + 2] cycloaddition of 2-(2-oxo-alkyl)benzketones with alkynes or enols (generated in situ from alkyl aldehydes or alkyl ketones) is described. A variety of poly-substituted naphthalene derivatives could be synthesized in up to 90% yield under mild reaction conditions. Poly-substituted 1,2-dihydronaphthalene derivatives could be obtained instead through Zn(II)-catalyzed cyclization of 2-(2-oxo-alkyl)benzketones with alkenes. Furthermore, the Fe(III)-catalyzed intramolecular version could lead to isoquinoline derivatives in high yields.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...