Ruthenium-catalyzed dynamic kinetic asymmetric transfer hydrogenation: stereoselective access to syn 2-(1,2,3,4-tetrahydro-1-isoquinolyl)ethanol derivatives†
Abstract
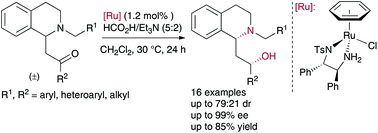
An efficient, stereoselective route to 2-(1,2,3,4-tetrahydro-1-isoquinolyl)ethanol derivatives through an asymmetric transfer hydrogenation of ring-substituted β-amino ketones via dynamic kinetic resolution has been developed. The reaction proceeded under mild conditions in the presence of RuCl[(S,S)-TsDPEN](benzene) and HCO2H/Et3N (5 : 2) as the hydrogen source, delivering in good yields a variety of syn 2-(1,2,3,4-tetrahydro-1-isoquinolyl)ethanol derivatives with excellent enantioselectivities (up to 99% ee) and diastereomeric ratios up to 71 : 29 dr.


 Please wait while we load your content...
Please wait while we load your content...