Highly meta-selective halogenation of 2-phenylpyridine with a ruthenium(i) catalyst†
Abstract
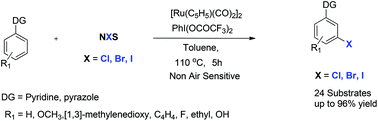
We report a concise, versatile, and practical method for PhI(OCOCF3)2 mediated direct meta-C–H monohalogenation of 2-phenylpyridine and its derivatives via an Ru(I)-catalyzed reaction. The significant advantage of this transformation is the creation of a highly site-selective C–X bond by using NXS as halogenating agents under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...