Copper-catalyzed carbene insertion into the sulfur–sulfur bond of benzenesulfonothioate†
Abstract
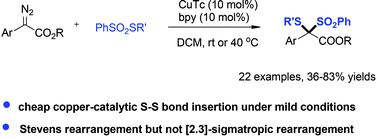
An unprecedented copper-catalyzed intermolecular sulfur–sulfur bond insertion between aryldiazoacetates and benzenesulfonothioate has been successfully developed. Diverse α-disulfur functionalized esters were synthesized in good to excellent yields. The sulfur ylide formation and subsequent Stevens rearrangement are considered as the key steps for the discovered reaction.


 Please wait while we load your content...
Please wait while we load your content...