Exploring the ESIPT dynamical processes of two novel chromophores: symmetrical structure CHC and asymmetric structure CHN†
Abstract
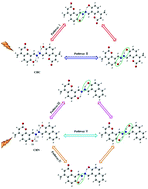
The detailed excited-state intramolecular proton transfer (ESIPT) dynamical processes of two novel chromophores, 8,8′-((1E,1E′)-hydrazine-1,2-diylidenebis(methanylylidene))bis-(7-hydroxy-4-methyl-2H-chromen-2-one) (CHC) (symmetrical structure) and 7-hydroxy-8-((E)-((E)-((2-hydroxynaphthalen-1-yl)-methylene)hydrazono)methyl)-4-methyl-2H-chromen-2-one (CHN) (asymmetric structure), which were synthesized in a previous study (Xiao et al., New. J. Chem., 2014, 38, 2386), were investigated by density functional theory (DFT) and time-dependent DFT (TDDFT) methods. Analysis of the bond lengths, angles and IR vibrational spectra confirmed that the intramolecular hydrogen bonds (HBs) of the CHC and CHN molecules were strengthened in the S1 state, which could facilitate ESIPT reactions. In addition, intramolecular charge transfer based on frontier molecular orbitals (MOs) and maps of the electron density difference between the S0 and S1 states indicated the possibility of ESIPT reactions for these two molecules. Moreover, to explore the detailed ESIPT dynamical processes of the CHC and CHN molecules, the potential energy surfaces (PESs) in the S0 and S1 states were constructed. The low excited-state potential barriers illustrated that both stepwise and simultaneous double ESIPT processes could occur for these two molecules. For the symmetrical structure CHC, two pathways of ESIPT processes existed as pathway I (stepwise double PT) and pathway II (simultaneous double PT). The relationship of the potential barriers was pathway II (4.71 kcal mol−1) < pathway I (6.15 and 7.62 kcal mol−1), which manifested that pathway II was more prone to double ESIPT for the CHC molecule. For the asymmetric structure CHN, three pathways of ESIPT processes existed as pathway III (stepwise double PT: firstly H8 from O7 to N9, and secondly H11 from O10 to N12), pathway IV (other stepwise double PT: firstly H11 from O10 to N12, and secondly H8 from O7 to N9) and pathway V (double PT). The relationship of the potential barriers was pathway III (2.67 and 6.75 kcal mol−1) < pathway IV (3.04 and 7.24 kcal mol−1) < pathway V (7.69 kcal mol−1), which indicated that pathway III was more susceptible to double ESIPT for the CHN molecule. Obviously, the ESIPT processes of the asymmetric structure CHN were more complicated than those of the symmetrical structure CHC.


 Please wait while we load your content...
Please wait while we load your content...