Transition metal-free aminofluorination of β,γ-unsaturated hydrazones: base-controlled regioselective synthesis of fluorinated dihydropyrazole and tetrahydropyridazine derivatives†
Abstract
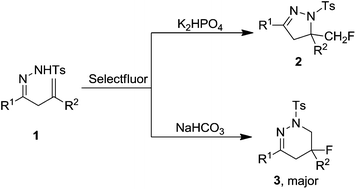
Various base-controlled regioselective reactions of β,γ-unsaturated hydrazones with Selectfluor were achieved under transition metal-free conditions. In the presence of K2HPO4 or NaHCO3, the intramolecular aminofluorination reaction took place readily to give the corresponding monofluoromethylated dihydropyrazoles or fluoro-tetrahydropyridazines, respectively. The possible pathways were proposed based on the experimental results.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...