Cycloaddition/annulation strategies for the construction of multisubstituted pyrrolidines and their applications in natural product synthesis†
Abstract
Pyrrolidines are privileged substructures of numerous bioactive natural products and drugs. How to synthesize these important synthetic targets in a more efficient manner is a hot research topic. Plenty of novel strategies and methods have been developed in recent years. Among them, cycloaddition and annulation strategies are the most efficient methods for the construction of multisubstituted pyrrolidines in terms of atom economy, stereoselectivity, diversity of products, and especially, their potential for asymmetric synthesis. These advantages have been well demonstrated by their applications in the syntheses of pyrrolidine-containing alkaloids. In this review, we highlight both the reaction and strategy development and synthetic applications from 2008 to June of 2017. This review covers 1,3-dipolar cycloadditions, formal [3 + 2] cycloadditions/annulations, and other interesting cycloaddition or annulation strategies to illustrate a so-called “reaction-based” strategy for the total synthesis of related natural products.
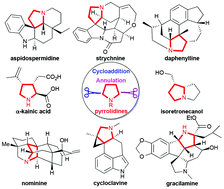
- This article is part of the themed collection: 2018 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...