Cobalt-catalyzed asymmetric reactions of heterobicyclic alkenes with in situ generated organozinc halides†
Abstract
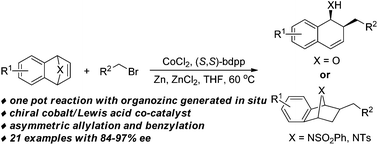
A general strategy for the asymmetric allylation and benzylation of heterobicyclic alkenes is described by employing in situ generated organozinc halides. This methodology features the application of a co-catalytic system comprising chiral cobalt complex and Lewis acid, which deliver both the asymmetric ring opening products of oxabenzonorbornadienes and the asymmetric addition products of azabenzonorbornadienes, respectively.


 Please wait while we load your content...
Please wait while we load your content...