An uncommon multicomponent reaction involving nucleophilic heterocyclic carbenes: facile synthesis of fully substituted cyclopentanones†
Abstract
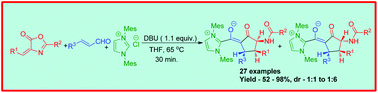
An unprecedented three component reaction involving arylidene oxazolone, enal and NHC, leading to the synthesis of fully substituted cyclopentanone derivatives with three contiguous stereocenters, is reported. This reaction illustrates the rare example of a multicomponent process involving NHCs, where the NHC-bound enolate is isolated by the interception of the NHC-homoenolate with arylidene oxazolone.


 Please wait while we load your content...
Please wait while we load your content...