Rh(iii)-Catalyzed regio- and stereoselective bisindolylation of vinyl acetate: an efficient approach toward (E)-1,2-bis(2-indolyl)ethenes†
Abstract
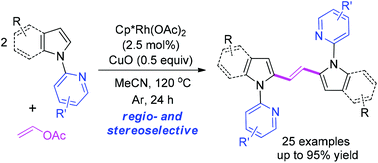
Vinyl acetate was employed as a cheap and convenient vinyl source in an Rh(III)-catalyzed C2-vinylation of indoles, which provides an efficient pathway toward a series of (E)-1,2-bis(2-indolyl)ethenes. Preliminary mechanism studies suggested that N-(2-pyridyl)-2-vinylindoles 2 and rhodacycle 4 were formed as key intermediates during the reaction process. The highly selective bisindolylation of vinyl acetate might be ascribed to the higher reactivity of vinylindoles 2 compared with vinyl acetate in a C–H olefination of N-(2-pyridyl)indoles.


 Please wait while we load your content...
Please wait while we load your content...