Carbonyl–olefin metathesis: a key review
Abstract
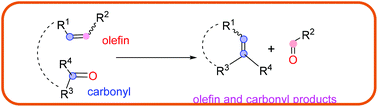
In organic chemistry, olefin–olefin metathesis of two unsaturated substrates for the formation of a new carbon–carbon bond has been widely explored and applied. Exploration of the construction of a carbon–carbon bond through the carbonyl–olefin metathesis reaction remains limited but has recently attracted significant interest and attention. This review covers most of the strategies involving metal-mediated, metal-free intramolecular, photochemical, Lewis acid-mediated, and catalytic carbonyl–olefin metathesis (e.g., ring-closing metathesis, ring-opening metathesis, and cross-metathesis). The necessity of stoichiometric amounts of transition metals with the synchronized formation of kinetically inert metal oxo species, which prevented the regeneration of the active metal catalyst, has most likely been the main reasons for the insignificance of the carbonyl–olefin metathesis reaction. Developing catalytic carbonyl–olefin metathesis is still a current challenge in organic synthesis.
- This article is part of the themed collection: 2018 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...