Chemoselective direct reductive trifluoromethylation of amides: a flexible access to functionalized α-trifluoromethylamines†
Abstract
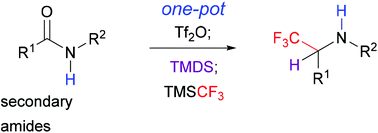
The direct transformation of amides is an emerging area in organic synthesis. Starting from common secondary amides, a novel and flexible approach to α-trifluoromethylamines is described. This one-pot method consists of the in situ activation of amides with triflic anhydride (Tf2O), partial reduction with 1,1,3,3-tetramethyldisiloxane (TMDS), and nucleophilic trifluoromethylation with trifluoromethyltrimethylsilane (TMSCF3). Thanks to the mild conditions, the reaction exhibited high chemoselectivity and good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...