Copper-catalyzed peri-selective direct sulfenylation of 1-naphthylamines with disulfides†
Abstract
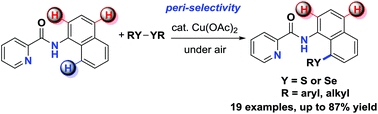
A copper-catalyzed peri-selective direct C–H sulfenylation of 1-naphthylamines with disulfides was developed. This protocol employs inexpensive Cu(OAc)2 as the catalyst, air as the terminal oxidant, and readily available diaryl (or dialkyl) disulfide and diselenide as chalcogenation reagents. High functional group tolerance and excellent regioselectivity were demonstrated by the efficient preparation of a wide range of 8-sulfenyl-1-naphthylamines.


 Please wait while we load your content...
Please wait while we load your content...