Pd-Catalyzed intramolecular C–H addition to the cyano-group: construction of functionalized 2,3-fused thiophene scaffolds†
Abstract
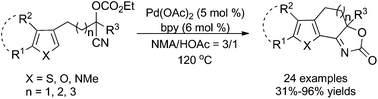
An efficient synthesis of 2,3-fused thiophenes was demonstrated through a Pd-catalyzed intramolecular C–H addition of thiophenes bearing cyanohydrin components at the C(3) positions to nitriles. A diversity of functionalized fused thiophenes with saturated six-, seven- or eight-membered rings can be prepared in good to excellent yields. This method can also be applied to prepare fused pyrrole and furan analogues through the use of different heteroarene scaffolds. Further demonstration of the synthetic utility of this Pd-catalyzed cyclization was explored.


 Please wait while we load your content...
Please wait while we load your content...