Visible-light-induced iminyl radical formation via electron-donor–acceptor complexes: a photocatalyst-free approach to phenanthridines and quinolines†
Abstract
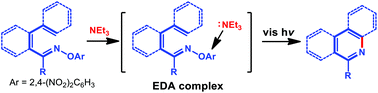
A visible light-induced synthesis of nitrogen-containing arenes from O-2,4-dinitrophenyl oximes has been reported. This photochemical strategy is photocatalyst-free and enabled by electron-donor–acceptor (EDA) complexes of O-2,4-dinitrophenyl oximes and Et3N. A variety of highly functionalized nitrogen-containing arenes, including 15 phenanthridines and 8 quinolines, were synthesized in excellent yields.


 Please wait while we load your content...
Please wait while we load your content...