Enantioselective total synthesis of periconiasin A†
Abstract
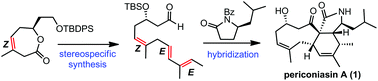
A concise, enantioselective total synthesis of periconiasin A, a hybrid natural product, has been achieved, featuring a ring closing metathesis (RCM) reaction and a Suzuki coupling to secure a (Z,E,E)-skipped triene system and an intramolecular Diels–Alder (IMDA) reaction to construct the challenging 6–9 fused ring system.


 Please wait while we load your content...
Please wait while we load your content...