Gold-catalyzed preparation of annelated 2-azetidinones via divergent heterocyclization of enyne-tethered oxazolidines†
Abstract
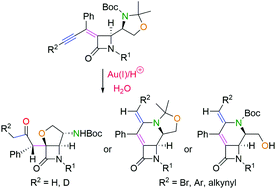
The divergent and selective syntheses of two types of annelated β-lactams, namely, furan- and tetrahydropyridine-fused 2-azetidinones, have been accomplished directly from 2-azetidinone-tethered oxazolidine-enynes through gold catalysis. The decisive effect of the presence or absence of substituents at the enyne end has been disclosed. The gold-catalyzed conditions are mild enough to tolerate the selective formation of condensed β-lactams without the erosion of the stereochemical integrity of the products and without damaging the sensitive 2-azetidinone nucleus.


 Please wait while we load your content...
Please wait while we load your content...