Palladium-catalyzed direct C–H ethoxycarbonylation of 2-aryl-1,2,3-triazoles and efficient synthesis of suvorexant†
Abstract
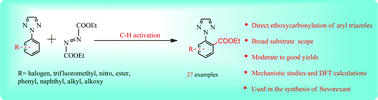
Efficient palladium-catalyzed C–H ethoxycarbonylation of 2-aryl-1,2,3-triazoles was developed by using diethyl azodicarboxylate as the esterification reagent. A wide variety of aryl esters containing 1,2,3-triazoles were obtained in moderate to good yields. In addition, density functional theory calculations were used to enhance the mechanistic studies.


 Please wait while we load your content...
Please wait while we load your content...