Copper-catalyzed coupling of oxime acetates and aryldiazonium salts: an azide-free strategy toward N-2-aryl-1,2,3-triazoles†
Abstract
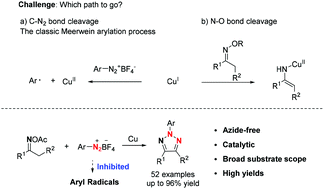
An azide-free strategy for the synthesis of N-2-aryl-1,2,3-triazoles via copper-catalyzed coupling of oxime acetates and aryldiazonium tetrafluoroborates is reported. With the magic p-methoxyphenyldiazonium tetrafluoroborate as the reaction partner, this copper-catalyzed system favored the desired single electron transfer process via N–O bond cleavage of oxime acetates, while the classic single electron transfer process via C–N2 bond cleavage of aryldiazonium tetrafluoroborates was sufficiently inhibited. Significantly, various oxime acetates were suitable for this reaction, delivering the corresponding 4-substituted-N-2-aryl-1,2,3-triazoles and 4,5-disubstituted-N-2-aryl-1,2,3-triazoles in high yield. Primary mechanism studies indicated that this reaction involved a radical procedure. Furthermore, various novel and attractive tetraphenylethylene and carbazole based N-2-aryl-1,2,3-triazoles were synthesized smoothly.


 Please wait while we load your content...
Please wait while we load your content...