A copper-catalyzed sulfonylative C–H bond functionalization from sulfur dioxide and aryldiazonium tetrafluoroborates†
Abstract
Sulfonylative C–H bond functionalization through a copper-catalyzed three-component reaction of 8-aminoquinoline amides, DABCO·(SO2)2 and aryldiazonium tetrafluoroborates is developed. Excellent selectivity in the para-position is observed for this copper-catalyzed transformation. This reaction is triggered by a copper-chelated complex via the coordination of the copper catalyst with the substrate and arylsulfonyl radical generated in situ, thus providing 5-sulfonyl-8-aminoquinoline amides in moderate to good yields.
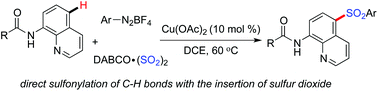
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...