Cu(i)-Catalyzed amidation/imidation of N-arylglycine ester derivatives via C–N coupling under mild conditions†
Abstract
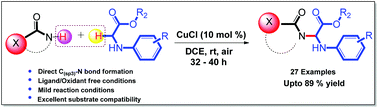
A mild and efficient reaction protocol for the cross-dehydrogenative carbon–nitrogen (CDCN) coupling of N-arylglycine ester derivatives with benzamides, aromatic heterocyclic amides (oxindole, isatins), acyclic and cyclic amides (lactams) and imides (phthalimide, succinimide) has been achieved. The copper-catalyzed C–N coupling reaction exhibits a wide substrate scope with respect to N-arylglycine ester derivatives. The ligand/oxidant free reaction facilitates the direct formation of a C(sp3)–N bond with high atom economy to furnish the corresponding amidated/imidated N-arylglycine ester derivatives in good to excellent yields under open flask conditions.


 Please wait while we load your content...
Please wait while we load your content...