A versatile rhodium(iii) catalyst for direct acyloxylation of aryl and alkenyl C–H bonds with carboxylic acids†
Abstract
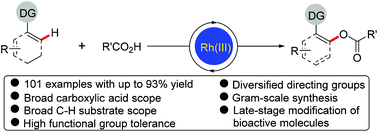
Rh(III)-Catalyzed highly regioselective direct acyloxylation of sp2 C–H bonds with carboxylic acids has been developed. The catalytic system consisting of a cationic Rh(III) complex and a silver oxidant allowed a variety of arenes and alkenes bearing different directing groups (not limited to strongly coordinating N-heterocyclic directing groups) to undergo efficient acyloxylation with a broad range of readily available alkyl, alkenyl and aryl carboxylic acids, and a number of valuable functional groups in both coupling partners were well tolerated in the reaction regardless of their electronic properties and positions. This method provides a straightforward and convenient access to various valuable acyloxylated products. Furthermore, the synthetic utility of this protocol was demonstrated by the later-stage functionalization and modification of biologically active compounds. Mechanistic studies reveal the involvement of five-membered rhodacycles as key intermediates.


 Please wait while we load your content...
Please wait while we load your content...