A new approach to thiazoloisoindigo and derivatives using a lithium tetramethylpiperidine promoted cyclization to thiazoloisatin†
Abstract
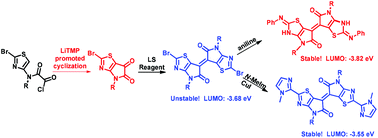
Brominated thiazoloisoindigo was synthesized for the first time from thiazoloisatin, which was obtained via an unprecedented nucleophilic cyclization strategy. Thiazoloisoindigo was shown to exhibit a deeper LUMO energy level than isoindigo and thienoisoindigo, which makes it a potential building block for n-type semiconductors. Moreover, brominated thiazoloisoindigo is sensitive toward nucleophilic attack, and its reaction with aniline resulted in an unexpectedly much more stable acceptor with a LUMO energy level as low as −3.82 eV.


 Please wait while we load your content...
Please wait while we load your content...