Enantioselective palladium-catalyzed C–H functionalization of pyrroles using an axially chiral 2,2′-bipyridine ligand†
Abstract
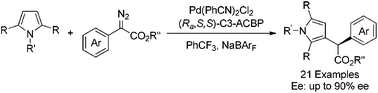
An enantioselective C–H functionalization of pyrrole derivatives with diazo compounds has been successfully realized with up to 90% ee by employing dichlorobis(benzonitrile)palladium with an axially chiral bipyridine ligand C3-ACBP as the catalyst. Asymmetric C–H functionalization at the gram scale was also conducted smoothly with good reactivity and enantioselectivity.
- This article is part of the themed collections: Organic Chemistry Frontiers HOT articles for 2018 and Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...