Total syntheses of pyrroloazocine indole alkaloids: challenges and reaction discovery
Abstract
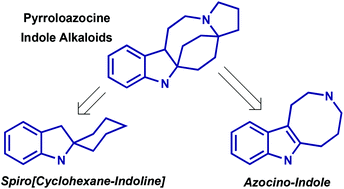
Lapidilectines, grandilodines, lundurines and tenuisines are indole alkaloids, isolated from plants of Kopsia genus. They feature a common indole-fused pyrroloazocine core, whose construction poses a significant synthetic challenge. In this review, we discuss the reported strategies for the total synthesis of this family of alkaloids with a focus on the different methods used for the construction of spiro[cyclohexane-2-indoline] and indole-pyrroloazocine intermediates, introduction of indole-fused cyclopropane as well as other new methodologies uncovered in the course of the total syntheses. In closing, the existing hypothesis of the biosynthetic origin and relationships of the pyrroloazocine indole alkaloids are presented.
- This article is part of the themed collections: 2018 Organic Chemistry Frontiers Review-type Articles, 7th EuCheMS Chemistry Congress – Molecular frontiers and global challenges and Synthetic Approaches to Natural Products via Catalytic Processes


 Please wait while we load your content...
Please wait while we load your content...