Asymmetric synthesis of 3-aminodihydrocoumarins via the chiral guanidine catalyzed cascade reaction of azlactones†
Abstract
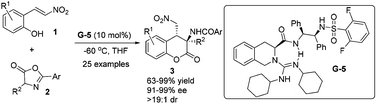
A highly efficient bifunctional guanidine catalyst was developed for the asymmetric cascade reaction between 2-nitrovinylphenols and azlactones. A wide variety of 3-aminodihydrocoumarin derivatives could be obtained in high yields (up to 99% yield) with excellent enantioselectivities (up to 99% ee) and diastereoselectivities (>19 : 1 dr).


 Please wait while we load your content...
Please wait while we load your content...