Tunable Cu(i)-catalyzed site-selective dehydrogenative amination of β,γ-unsaturated hydrazones for divergent synthesis of pyrazol-4-ones and 1,6-dihydropyradazines†
Abstract
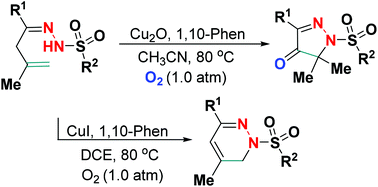
A new tunable Cu(I)-catalyzed site-selective dehydrogenative amination of β,γ-unsaturated hydrazones has been established using readily accessible dioxygen as a green oxidant. The presence of Cu2O/O2 regiospecifically accessed densely functionalized pyrazol-4-ones with one quaternary carbon-amino functionality, in which O2 acted as both a reaction component and an oxidant, whereas the employment of CuI/O2 as a catalytic oxidation system resulted in 1,6-dihydropyradazines with good yields.


 Please wait while we load your content...
Please wait while we load your content...