Cross-coupling of sulfonic acid derivatives via aryl-radical transfer (ART) using TTMSS or photoredox†
Abstract
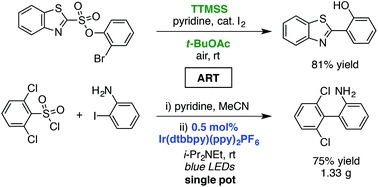
The intramolecular cross-coupling of sulfonic acid derivatives occurs in the presence of tris(trimethylsilyl)silane (TTMSS) at room temperature and in air to form biaryl compounds. A photoredox-catalyzed procedure is also described. These protocols provide mild and convenient alternatives to standard tin-mediated reactions. Combined with the trivial preparation of the substrates from activated sulfonic acids and 2-halophenols or anilines, this work presents a useful means to employ sulfonic acid derivatives in cross-coupling transformations. A modified linker to realize high regioselectivity is also presented. Finally, a one-pot cross-coupling procedure is demonstrated.


 Please wait while we load your content...
Please wait while we load your content...