Gold(i) catalyzed cascade cyclization: intramolecular two-fold nucleophilic addition to vinylidenecyclopropanes (VDCPs)†
Abstract
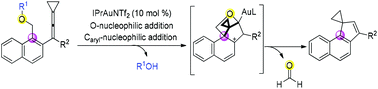
A gold-catalyzed cascade cyclization of vinylidenecyclopropanes (VDCPs) bearing a naphthyl moiety and an ortho-alkoxylmethyl group has been reported in this paper, affording spiro[cyclopenta[a]naphthalene-1,1′-cyclopropanes] in moderate to good yields. The reaction proceeded through a two-fold intramolecular nucleophilic addition (O-nucleophilic addition and then Caryl-nucleophilic addition) to a vinylidenecyclopropane moiety to give a highly strained intermediate and subsequent retro-Diels–Alder addition. The reaction mechanism has been investigated on the basis of a deuterium labeling experiment, mass spectrometric investigation and DFT calculation.


 Please wait while we load your content...
Please wait while we load your content...