Organocopper triggered cyclization of conjugated dienynes via tandem SN2′/Alder-ene reaction†
Abstract
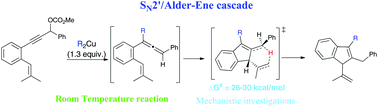
Propargylic carbonates were converted to indenes through a SN2′/Alder-ene cascade triggered by organocopper reagents. The reaction tolerates different organocopper species generated either from organolithiums or Grignard reagents. A catalytic version of this strategy could be devised using either copper or iron catalysts. Attempts to transfer chirality from an enantioenriched substrate revealed a moderate chirality conversion because of a low discrimination between the two faces of the internal olefinic partner with these typical substrates. The theoretical investigation supports a concerted closed-shell mechanism and highlights the influence of the substituents on the activation parameters and on the synchronicity of C–H bond breaking and C–C bond forming during the Alder-ene step.


 Please wait while we load your content...
Please wait while we load your content...