A direct synthetic method for (nitronyl nitroxide)-substituted π-electronic compounds via a palladium-catalyzed cross-coupling reaction with a zinc complex†
Abstract
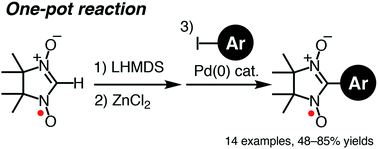
We have developed an efficient synthetic method for (nitronyl nitroxide)-substituted (NN-substituted) π-electronic compounds via palladium-catalyzed cross-coupling reactions with a zinc complex of the parent nitronyl nitroxide radical anion. Various aryl iodides can be directly converted to the desired coupling compounds with high efficiency. The utility of the present method has been demonstrated by direct synthesis of a newly prepared radical species, NN-substituted phenothiazine 15P, which could not be obtained from the corresponding aldehyde compound by typical condensation-oxidation methods. The molecular structure and characteristic electronic nature of 15P were examined by single-crystal X-ray diffraction analysis and cyclic voltammetry, indicating that 15P is a promising candidate as a precursor for a triplet ground state species by one-electron oxidation.
- This article is part of the themed collection: Pi conjugated system bricolage (figuration) toward functional organic molecular systems


 Please wait while we load your content...
Please wait while we load your content...