Synthesis and properties of open-cage fullerene C60 derivatives: impact of the extended π-conjugation†
Abstract
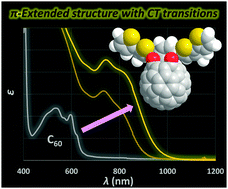
We have developed a method for the synthesis of open-cage fullerene C60 derivatives with extended π-conjugation bearing thienyl groups. By applying this method to an asymmetric diketo derivative, the symmetric form can be obtained without changing the molecular formula. To investigate the structure–property relationship for the asymmetric and symmetric forms, we conducted electrochemical and photophysical measurements. The UV-vis absorption edge was shifted by 210 nm upon changing from the asymmetric to the symmetric form due to the narrower HOMO–LUMO gap, which was also demonstrated by electrochemical analyses. From theoretical calculations, the major contribution of the longest wavelength absorption for the symmetric form is assignable to unusual intramolecular charge transfer transitions whereas π–π* transitions are dominant for the asymmetric form.
- This article is part of the themed collections: 2018 Materials Chemistry Frontiers Review-type Articles and Pi conjugated system bricolage (figuration) toward functional organic molecular systems


 Please wait while we load your content...
Please wait while we load your content...