Enhanced hydrogen evolution of MoS2/RGO: vanadium, nitrogen dopants triggered new active sites and expanded interlayer†
Abstract
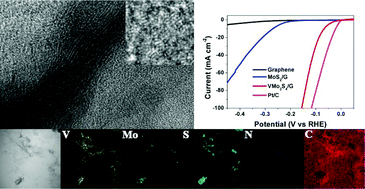
Layered MoS2 has been recognized as a promising low-cost alternative to Pt-based electrocatalysts towards the hydrogen evolution reaction (HER). Intensive interest has been mainly focused on designing MoS2 nanostructures with large amounts of active edge sites and fast charge transfer. Here we report the synthesis of vanadium and nitrogen co-doped MoS2 on reduced graphene oxide with new defect sites on the basal/edge planes and expanded interlayer spacing, which shows remarkable catalytic merits with an extremely low overpotential of 68 mV at 10 mA cm−2 and a Tafel slope of 41 mV dec−1. Its performance is superior to most current MoS2 electrocatalysts. In addition to the charge transfer benefits of the RGO substrate, the vanadium and nitrogen dopants trigger defect sites on the basal/edge planes, and an optimized electronic structure and expanded interlayer distance are also responsible for the enhancement in catalysis. This contribution may offer a potential way to design advanced MoS2 catalysts by modulating the defect sites and interlayer distance for efficient electrochemical water splitting.


 Please wait while we load your content...
Please wait while we load your content...