Micro-structure studies of the molten binary K3AlF6–Al2O3 system by in situ high temperature Raman spectroscopy and theoretical simulation
Abstract
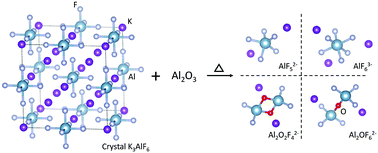
In order to assist in predicting the properties of molten cryolite-alumina electrolytes widely used in the Hall–Héroult process, the micro-structure of the molten binary system was investigated by in situ high temperature Raman spectroscopy from room temperature to 1273 K under an argon atmosphere. The symmetric stretching vibrational wavenumbers of possible clusters in the molten system were further calculated through quantum chemistry ab initio simulations and compared with the experimental Raman spectra of the molten system. The molten K3AlF6–Al2O3 system was found to consist of [AlF5]2−, [AlF6]3−, [Al2OF6]2− and [Al2O2F4]2− clusters. With the increasing addition of Al2O3 to molten K3AlF6, the structure of six-coordinated Al atoms connected by terminal fluorine is partly believed to evolve into a structure of two four-coordinated Al atoms connected by single-bridging oxygen. The mole percentage of the species in the melt was also calculated from the experimental Raman spectra. As the Al2O3 content increased from 0 to 8.0 wt%, the content of [Al2OF6]2− in the melt was found to increase swiftly from 0 to 33.0 mol%, while the [Al2O2F4]2− content decreased after reaching a maximum of 1.5 mol%.


 Please wait while we load your content...
Please wait while we load your content...