High-performance rechargeable aqueous Zn-ion batteries with a poly(benzoquinonyl sulfide) cathode†
Abstract
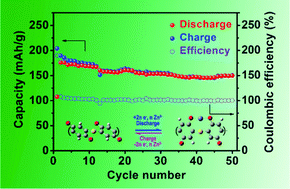
We report high-performance aqueous Zn-ion batteries consisting of a zinc anode, a poly(benzoquinonyl sulfide) (PBQS) cathode, and a 3 M Zn(CF3SO3)2 aqueous electrolyte. The PBQS cathode displays an initial discharge capacity of 203 mA h g−1 at 0.1C and a good capacity retention of 86% after 50 cycles at 0.2C. The PBQS cathode can deliver a high reversible capacity of 126 mA h g−1 at a high rate of 5.0C. Meanwhile, we also studied the redox mechanism during discharge/charge processes by ex situ infrared spectra and theoretical calculations, revealing that reversible bonding of Zn2+ ions with carbonyl oxygen atoms in PBQS occurs in the redox reactions of PBQS molecules. The redox reactions of PBQS molecules can be regarded as the reversible bonding of Zn2+ ions with carbonyl oxygen atoms in PBQS.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...