Phase equilibria and crystal structure relationships in the ternary Li–B–C system
Abstract
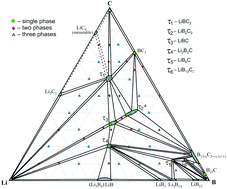
Phase equilibria in the Li–B–C ternary system have been investigated by X-ray powder diffraction, scanning electron microscopy, electron probe microanalysis and differential scanning calorimetry. The isothermal section of the phase diagram has been constructed over the whole concentration range at 400 °C. The Li–B–C system is characterized by the formation of six ternary compounds: τ1 – LiBC3, τ2 – LiB2C2, τ3 – LiBC, τ4 – Li2B2C, τ5 – LiB6C and τ6 – LiB13C2. The formation of the new ternary compound LiB2C2 (own structure type, space group P63/mmc, a = 2.5930(3) Å, c = 22.680(1) Å) and binary BC3 (space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2, a = 2.4588(4) Å, c = 6.770(2) Å) has been revealed. The crystal structures are discussed in the light of the underlying graphene and heterographene networks.
m2, a = 2.4588(4) Å, c = 6.770(2) Å) has been revealed. The crystal structures are discussed in the light of the underlying graphene and heterographene networks.


 Please wait while we load your content...
Please wait while we load your content...