Unlocking the role of MgO in the carbonation of alkali-activated slag cement
Abstract
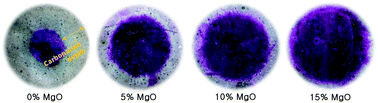
The present study investigates the role of MgO in local structural changes occurring during the accelerated carbonation of alkali-activated slag (AAS) using synchrotron X-ray diffraction, mercury intrusion porosimetry, thermogravimetry, and nuclear magnetic resonance spectroscopy. The obtained results provide new insight showing that MgO incorporation into AAS reduced Al substitution in C-S-H and led to the formation of secondary phases, which significantly altered the route of carbonation. In particular, the carbonation of secondary phases (i.e., layered double hydroxide) occurred and reduced the extent of carbonation in C-A-S-H. Consequently, a buffer-like system is provided, showing similar behavior but different principles involved in comparison with those in Portland cement. This effect was found to dramatically reduce the degrees of decalcification, dehydration and polymerization of C-A-S-H under accelerated carbonation conditions.


 Please wait while we load your content...
Please wait while we load your content...