A novel N-methylimidazolium-based poly(ionic liquid) to recover trace tetrachloroaurate from aqueous solution based on multiple supramolecular interactions†
Abstract
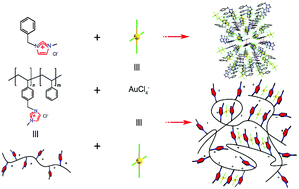
Two novel ionic liquids, [Bzmim]Cl and [Bzmim][AuCl4], were successfully synthesized and characterized by 1H NMR spectroscopy, single crystal structure analysis and quantum mechanical calculations. Meanwhile, multiple supramolecular interactions of [Bzmim][AuCl4] (hydrogen bonds, Cl⋯π, Au⋯π interactions and π–π stacking) were found between the N-methylimidazolium cation and AuCl4−. Interestingly, the interactions made it easy for [Bzmim][AuCl4] to form a 3D supramolecular structure. Thus, a series of N-methylimidazolium-based poly(ionic liquids) were synthesized to recover Au(III) in aqueous solution. They show high adsorption capacity and excellent selectivity for tetrachloroaurate anions which can be used as solid phase extraction agents over a wide temperature range. Notably, the acid thiourea can desorb Au(III) from polymeric ionic liquids (PILs), thus they also exhibit excellent recyclability. Among them, PS-b-PVBnMeImCl-5.9 has the highest efficiency for Au(III) recovery after five adsorption–desorption cycles. Compared with the Raman spectroscopy results of gold-containing PS-b-PVBnMeImCl-5.9, 1H NMR of [Bzmim][AuCl4] revealed that the Au(III) recovery mechanism by polymeric ionic liquids was due to a combination of electrostatic interactions and multiple weak intermolecular interactions.


 Please wait while we load your content...
Please wait while we load your content...