Facile and cost-effective growth of a highly efficient MgCo2O4 electrocatalyst for methanol oxidation†
Abstract
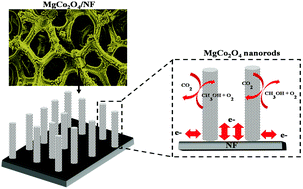
A MgCo2O4 based electrode is employed as a novel electrocatalyst material for the electrochemical oxidation of methanol. The binder-free and carbon-free spinel MgCo2O4 nanorod-like structures were grown directly on nickel foam (NF) using a facile and cost-effective co-precipitation method followed by calcination. Cyclic voltammetry (CV), chronoamperometry (CA) and electrochemical impedance spectroscopy (EIS) tests were used to study the electrocatalytic performance of the MgCo2O4/NF electrode. The MgCo2O4/NF electrode exhibits higher electrocatalytic activity, a lower onset potential and superior stability when tested as an electrocatalyst for methanol electrooxidation. The high surface area and large pore volume of MgCo2O4 with a mesoporous structure offer higher numbers of active sites for methanol electrooxidation. The higher catalytic activity and stability, along with the facile and scalable synthesis route are promising features for the use of cheap non-Pt based catalyst materials for methanol electrooxidation.


 Please wait while we load your content...
Please wait while we load your content...