Selective CO2 adsorption in water-stable alkaline-earth based metal–organic frameworks†
Abstract
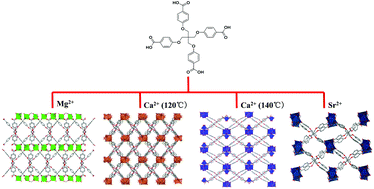
Four novel metal–organic frameworks (MOFs) built from alkaline-earth metal ions and the flexible tetrahedral carboxylate ligand tetrakis[4-(carboxyphenyl)oxamethyl]methane acid (H4L) were synthesized using solvothermal methods. A variety of three-dimensional frameworks were obtained when employing different alkaline earth ions with the formula [Mg2(L)(H2O)(DMA)]·DMA (1), [Ca4(L)2(DMA)3] (2), [Ca4(L)2(H2O)2(DMA)2]·(3DMA) (3) and [Sr4(L)2(DMF)4]·(2DMF) (4) reflecting the variation in the ionic radius of alkaline-earth ions as well as the key role of the synthetic conditions used. By removing the guest molecules, a framework shrinking was observed driven by the structural flexibility of the H4L ligand. This resulted in large diffusional resistances towards N2 over CO2 molecules, therefore leading to a good CO2/N2 separation selectivity. Both Ca-based MOFs were very stable up to 98% relative humidity, while Mg- and Sr-based MOFs were much less stable.


 Please wait while we load your content...
Please wait while we load your content...