Nanocomposite of MoO2 and MoC loaded on porous carbon as an efficient electrocatalyst for hydrogen evolution reaction†
Abstract
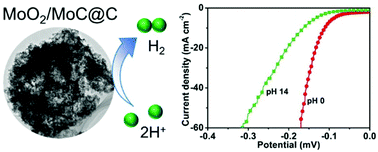
The development of an effective, non-precious electrocatalyst for hydrogen evolution reaction (HER) is highly desirable for electrocatalytic water splitting and electrochemical/photoelectrochemical water splitting to produce hydrogen. Herein, a facile one-step ultrasonic spray pyrolysis method is developed for the synthesis of MoO2–MoC composite loaded on porous carbon (MoO2/MoC@C), and the MoO2/MoC@C is found to exhibit prominent catalytic activity in the HER. The overpotential required for a current density of 20 mA cm−2 is as small as 133 mV in 0.5 M H2SO4 and 203 mV in 1 M KOH for the MoO2/MoC@C, and the value is superiorly comparable to that of reported metal carbides. The MoO2/MoC@C can work stably both in acidic and basic solution over 30 h with 95% faradaic efficiency in 0.5 M H2SO4 and 93% in 1 M KOH. The synergic effect between MoO2 and MoC is suggested by density functional theory calculations.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...