Chiral metal–organic frameworks constructed from four-fold helical chain SBUs for enantioselective recognition of α-hydroxy/amino acids†
Abstract
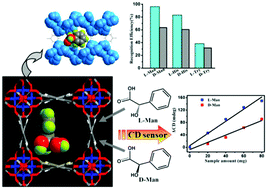
The chiral recognition of racemic α-hydroxy/amino acids is an essential and challenging mission because enantiomers may profoundly differ in biological function, pharmacology and toxicity. Three stable, chiral 3D metal–carboxylate frameworks, namely [M2(bptc)(H2O)(MeOH)]·3H2O (bptc4− = 3,3′,5,5′-biphenyltetracarboxylate, M = Co, CoNi for 1–2, and [Ni2(bptc)(MeOH)2]·3H2O for 3) have been successfully obtained by spontaneous resolution with an achiral ligand H4bptc, and they crystallize in the chiral tetragonal space group I4122, and feature chiral four-fold helical metal chains as SBUs. In particular, the Co-MOF material could be used to rapidly and sensitively recognize racemic α-hydroxy/amino acids by the intensity change of circular dichroism signals. A large relative difference of 38.59% in circular dichroism signals for D/L-mandelic acid is achieved, which may be ascribed to the specific recognition sites (i.e., groove of helical chains) and different bonding energies of D/L-isomers in the chiral microenvironment of the crystal structure.


 Please wait while we load your content...
Please wait while we load your content...