Phosphazene-catalyzed oxa-Michael addition click polymerization†
Abstract
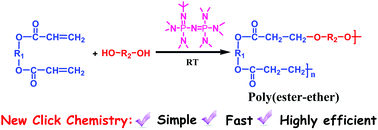
This paper reports a new type of click chemistry via a phosphazene bases-catalyzed oxa-Michael addition of an alcohol to an acrylate. The studies on the reactions between small molecules showed that only 5 mol% phosphazene base, t-BuP2, could catalyze the reaction between the acrylate double bonds and a primary alcohol and drive the reaction to completion within a few minutes under solvent-free conditions at room temperature. Additionally, t-BuP2 was a particularly efficient catalyst for the oxa-Michael addition of secondary alcohols to acrylate double bonds under similar conditions. Moreover, applying this reaction to the synthesis of polymers could successfully afford degradable poly(ester-ether)s with excellent monomer conversions under mild conditions. Throughout the polymerization, transesterification reactions were unavoidable, which increased the randomness in the structures of the resulting polymers. Considering the remarkable features of this method, such as the ready availability of the alcohol substrates, this work provides a simple, easy, and versatile method for synthesizing degradable polymers from commercially available compounds and will be useful in polymer chemistry.


 Please wait while we load your content...
Please wait while we load your content...