Hydrogen bonding reinforcement as a strategy to improve upper critical solution temperature of poly(N-acryloylglycinamide-co-methacrylic acid)†
Abstract
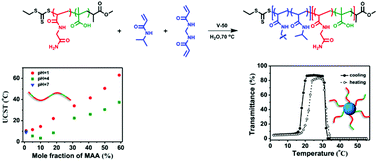
Hydrogen bonding reinforcement via copolymerization of N-acryloylglycinamide (NAGA) with methacrylic acid (MAA) is employed as a strategy to improve the upper critical solution temperature (UCST) to a biologically relevant range. P(NAGA-co-MAA) copolymers with MAA molar fraction in the range of 1–81 mol% are synthesized via reversible addition–fragmentation chain transfer (RAFT) polymerization. The UCST (3.5–37.5 °C) of the copolymers scales with MAA molar fraction in the range of 10–60 mol% when measured at pH 4 and 1 wt% concentration. Using a copolymer with a suitably high UCST as a macromolecular chain transfer agent, doubly thermoresponsive nanogels consisting of P(NAGA-co-MAA) copolymer as the shell and cross-linked poly(N-isopropylacrylamide) (PNIPAM) as the core are synthesized via RAFT aqueous dispersion polymerization. The nanogels show distinct thermal transitions with both upper and lower critical solution temperatures (UCST and LCST).


 Please wait while we load your content...
Please wait while we load your content...