ROS-responsive poly(ε-caprolactone) with pendent thioether and selenide motifs†
Abstract
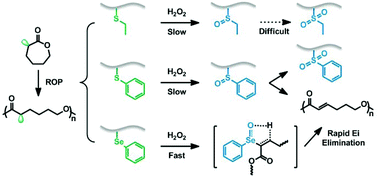
The chalcogen-containing polyesters represent an important branch of reactive oxygen species (ROS)-responsive biodegradable polymers. Herein, we report the synthesis and oxidation properties of three ROS-responsive poly(ε-caprolactone) (PCL)-type polyesters. Three new α-substituted caprolactone-type monomers: α-ethylthio caprolactone (M1), α-phenylthio caprolactone (M2), and α-phenylseleno caprolactone (M3) were synthesized. The controlled ring-opening polymerizations (ROP) of these three monomers were demonstrated with benzyl alcohol as the initiator and stannous octoate as the catalyst at 130 °C, affording three homopolymers (P1–P3) with low dispersities. M1 showed a faster ROP rate than M2 and M3. Using PEG as the macroinitiator, three kinds of amphiphilic block copolymers (PS1, PS2, and PSE) that could form micellar nanoparticles in water were also prepared. The oxidation profiles of these block copolymer nanoparticles in neutral phosphate buffer were investigated by 1H NMR, Nile red fluorescence, laser light scattering, and TEM. We found that the PS1 nanoparticles could sufficiently swell and finally became loosely associated water soluble polymers due to the H2O2-induced hydrophobic–hydrophilic transition from ethyl thioether to its sulfoxide. In contrast, the PS2 nanoparticles degraded very slowly owing to the strong hydrophobic nature of the phenyl thioether. Though the PSE nanoparticles responded to H2O2 much faster than both PS1 and PS2 because of the superior sensitivity of the selenide, the oxidatively produced block copolymer remained amphiphilic and the nanoparticles could not be dissociated completely. These results were discussed based on the results of H2O2-induced oxidation of three model compounds (T1–T3). All the copolymers showed negligible cytotoxicity with the polymer concentration up to 0.5 mg mL−1, and PS1 has potential as a ROS-responsive vehicle for photodynamic therapy.


 Please wait while we load your content...
Please wait while we load your content...