Synthesis of poly(3-hexylthiophene) based rod–coil conjugated block copolymers via photoinduced metal-free atom transfer radical polymerization†
Abstract
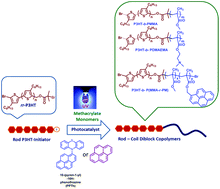
The photo-mediated “metal-free” atom transfer radical polymerization approach has been investigated for the first time to polymerize methyl methacrylate, N,N-dimethylamino-2-ethyl methacrylate, 2-([4,6-dichlorotriazin-2-yl]oxy)ethyl methacrylate and a mixture of methyl methacrylate and 1-pyrenemethyl methacrylate from a regioregular poly(3-hexylthiophene) (P3HT) macroinitiator to produce well-defined rod–coil conjugated diblock copolymers, highly relevant for use as organic electronic materials without metal catalyst contamination. This was achieved using pyrene and a pyrenyl N-substituted phenothiazine (PPTh) as UV light photoredox catalysts. Spectral and chromatographic results revealed that the applied synthetic approach generated P3HT-based diblock copolymers in a controlled manner and with narrow molecular weight distributions (Đ ≤ 1.3). From a comparison of the efficiency of the photocatalysts, PPTh has shown advantages over pyrene, including much lower catalyst content and higher polymerization conversions.


 Please wait while we load your content...
Please wait while we load your content...